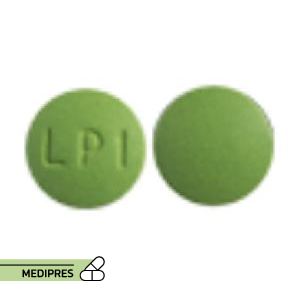
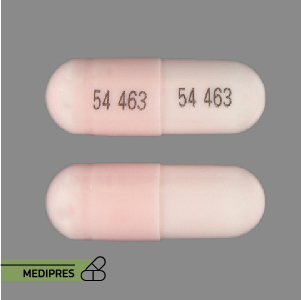
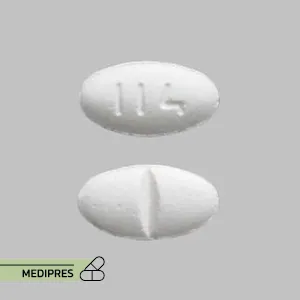
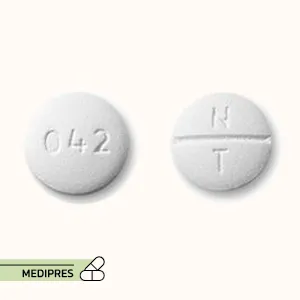
Lurasidone Tablets
23 June, 2023
Lysteda
23 June, 2023LYMEPAK
Generic name:
Amoxicillin and Clavulanate Potassium
Drug class:
Beta-lactam antibiotic combination (Penicillin class with beta-lactamase inhibitor)
Dosage form:
- Tablets (various strengths)
- Oral suspension
- Chewable tablets
Root of administration:
Oral
Dose:
- Adults: Typically 250 mg/125 mg to 875 mg/125 mg every 12 hours, or 500 mg/125 mg every 8 hours
- Children: Dose based on weight, generally 20-40 mg/kg/day of amoxicillin component divided every 8-12 hours
- Dosing frequency and duration vary based on infection type and severity
- Adjustments necessary for renal impairment
- Varies by indication; consult label
Mechanism of action:
Amoxicillin inhibits bacterial cell wall synthesis by binding to penicillin-binding proteins, leading to cell lysis and death. Clavulanate potassium is a beta-lactamase inhibitor that protects amoxicillin from degradation by beta-lactamase enzymes produced by resistant bacteria, expanding the spectrum of antibacterial activity.
Drug usage cases:
- Acute bacterial sinusitis
- Community-acquired pneumonia
- Acute otitis media
- Skin and soft tissue infections
- Urinary tract infections
- Animal bites and human bite wounds
- Intra-abdominal infections
- Dental infections
- Off-label: Some cases of Helicobacter pylori eradication as part of combination therapy
Drug contra indications:
- Known hypersensitivity to amoxicillin, penicillins, or other beta-lactam antibiotics
- History of severe allergic reaction (e.g., anaphylaxis) to cephalosporins or other beta-lactams
- Previous cholestatic jaundice or hepatic dysfunction associated with amoxicillin/clavulanate use
- Varies by indication; consult label
Side effects:
- Gastrointestinal upset: diarrhea, nausea, vomiting
- Clostridium difficile-associated diarrhea
- Allergic reactions: rash, urticaria, pruritus, anaphylaxis
- Hepatotoxicity: transient elevations in liver enzymes, cholestatic jaundice
- Hypersensitivity vasculitis
- Rare: Stevens-Johnson syndrome, toxic epidermal necrolysis
- Transient neutropenia, thrombocytopenia
- Oral candidiasis
- Headache, dizziness
- Varies by individual and dose; consult label for comprehensive listing
Warnings:
- Monitor for signs of allergic reactions, especially in patients with history of penicillin allergy
- Use with caution in patients with a history of liver disease or jaundice
- Discontinue use if severe diarrhea or signs of superinfection occur
- Monitor renal function; dosage adjustments needed in renal impairment
- Prolonged use may lead to overgrowth of non-susceptible organisms
- May cause hypersensitivity reactions which can be severe or life-threatening
- Use caution when administered concomitantly with oral anticoagulants
- Contains potassium clavulanate, may cause gastrointestinal intolerance at higher doses
- Varies by indication; consult label
Use during pregnancy or breastfeeding:
Amoxicillin/clavulanate is classified as Pregnancy Category B. Animal reproduction studies have not demonstrated a risk to the fetus, but there are no adequate and well-controlled studies in pregnant women. Use during pregnancy should be considered only if clearly needed and if benefits outweigh risks.
The drug is excreted in breast milk in small amounts; it is generally considered compatible with breastfeeding. However, infants should be monitored for diarrhea, candidiasis, or allergic reactions.
Consult physician before use during pregnancy or breastfeeding to weigh benefits and risks based on clinical situation.