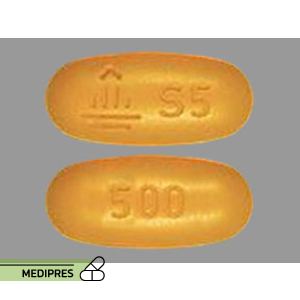
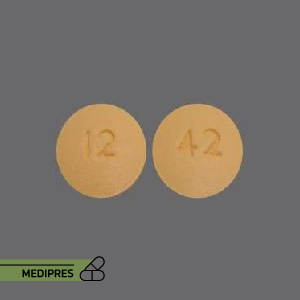
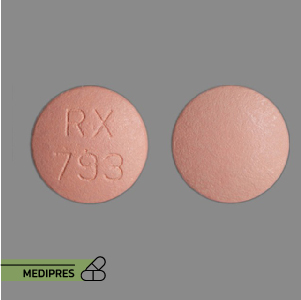
Sulfisoxazole
23 June, 2023
Sumatriptan (Transdermal)
23 June, 2023Sumatriptan
Generic name: Sumatriptan succinate
Dosage form: tablet, film coated
Drug class: Antimigraine agents
Brand Names: Imitrex, Imigran, Sumavel DosePro, Onzetra Xsail
Route of Administration: Oral, Intranasal, Subcutaneous
Dose: Tablets: 25 mg, 50 mg, 100 mg; Nasal spray: 5 mg, 20 mg; Injection: 4 mg/0.5 mL, 6 mg/0.5 mL; Nasal powder: 11 mg per device
Mechanism of Action:
Sumatriptan is a selective serotonin (5-HT1) receptor agonist that works by stimulating serotonin receptors in the brain, causing vasoconstriction of dilated blood vessels. This helps to relieve migraine headaches and cluster headaches. Sumatriptan also inhibits the release of certain neuropeptides that cause inflammation and pain, providing further relief from migraine symptoms.
Drug Usage Cases:
Sumatriptan is used to treat acute migraine attacks with or without aura in adults. It is also used to treat acute cluster headaches. It is not intended for the prevention of migraines or to reduce the number of migraine attacks. It should only be used when a clear diagnosis of migraine or cluster headache has been established by a healthcare provider.
Drug Contraindications:
Sumatriptan is contraindicated in patients with a history of hypersensitivity to sumatriptan or any of its components. It should not be used in patients with a history of certain cardiovascular conditions, including ischemic heart disease, Prinzmetal’s angina, uncontrolled hypertension, peripheral vascular disease, or history of stroke or transient ischemic attack (TIA). It is also contraindicated in patients with hemiplegic or basilar migraine, severe hepatic impairment, and those using other serotonin receptor agonists or monoamine oxidase inhibitors (MAOIs) within the past 2 weeks.
Side Effects:
Common side effects of sumatriptan may include dizziness, drowsiness, sensations of tingling or numbness, feeling warm or cold, pressure or tightness in the chest or throat, dry mouth, nausea, and muscle pain. Serious side effects that require immediate medical attention include:
Signs of a heart attack (chest pain or pressure, pain spreading to the jaw or shoulder, shortness of breath). Signs of a stroke (sudden numbness or weakness, especially on one side of the body; sudden severe headache, slurred speech, vision changes). Severe allergic reactions (rash, itching, hives, difficulty breathing, swelling of the face, lips, tongue, or throat). Changes in mental status, agitation, hallucinations, loss of coordination. Serotonin syndrome (agitation, hallucinations, fever, sweating, shivering, fast heart rate, muscle stiffness, twitching, loss of coordination, nausea, vomiting, diarrhea)
Warnings:
Sumatriptan may cause serious cardiovascular side effects, including heart attacks, arrhythmias, stroke, and death. It should be used with caution in patients with risk factors for heart disease, such as high blood pressure, high cholesterol, diabetes, smoking, obesity, or a family history of heart disease. Sumatriptan may cause an increase in blood pressure, so monitoring is essential for patients with hypertension. It is not recommended for use in patients with severe hepatic impairment. Patients should be warned about the risk of serotonin syndrome, especially if used with other serotonergic drugs.
Use During Pregnancy or Breastfeeding:
Sumatriptan is categorized as pregnancy category C, meaning that risk to the fetus cannot be ruled out. It should be used during pregnancy only if the potential benefits justify the potential risk to the fetus. Sumatriptan is excreted in human breast milk. Nursing mothers should consult their healthcare provider before using this medication and consider discontinuing breastfeeding for 12 hours after taking the medication to minimize infant exposure.