Vitamin B12 Injection
23 June, 2023
Vivlodex
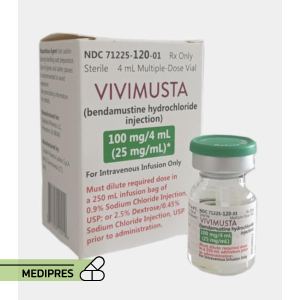
23 June, 2023Vivimusta
Generic name: Bendamustine hydrochloride
Drug class: Alkylating agent, Nitrogen mustard analogue
Dosage form: Injection
Root of administration: Intravenous
Dose: The recommended dose for chronic lymphocytic leukemia (CLL) is 100 mg/m² administered intravenously on days 1 and 2 of a 28-day cycle. For indolent B-cell non-Hodgkin lymphoma (NHL), the recommended dose is 120 mg/m² administered intravenously on days 1 and 2 of a 28-day cycle.
Mechanism of action: Bendamustine hydrochloride is an alkylating agent that cross-links DNA strands, leading to impaired DNA synthesis and repair, resulting in cell death.
Drug usage cases:
- Treatment of chronic lymphocytic leukemia (CLL)
- Treatment of indolent B-cell non-Hodgkin lymphoma (NHL)
Drug contraindications:
- Hypersensitivity to bendamustine hydrochloride or any component of the formulation
- Severe bone marrow suppression
- Active infections
Side effects:
- Myelosuppression (e.g., neutropenia, thrombocytopenia, anemia)
- Nausea and vomiting
- Fatigue
- Fever
- Infections
- Hypersensitivity reactions (e.g., rash, fever, chills)
- Elevated liver enzymes
- Electrolyte disturbances
Warnings:
- Monitor complete blood counts regularly due to risk of myelosuppression
- Use caution in patients with hepatic impairment
- Avoid use in patients with active infections
- Ensure adequate hydration to reduce risk of tumor lysis syndrome
- Handle and dispose of the drug according to hazardous drug handling guidelines
Use during pregnancy or breastfeeding: Bendamustine hydrochloride is classified as a Category D drug, indicating positive evidence of human fetal risk. It should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus. Women of childbearing potential should use effective contraception during treatment and for at least 6 months after the last dose. It is not known whether bendamustine is excreted in human milk; therefore, breastfeeding is not recommended during treatment and for at least 6 months after the last dose.