Lorazepam
23 June, 2023
Macrobid
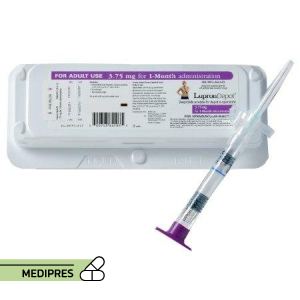
23 June, 2023Lupron
Generic name: leuprolide acetate
Drug class: Gonadotropin-releasing hormone (GnRH) agonist
Dosage form: Injectable suspension (depot formulation)
Root of administration: Intramuscular (IM) or subcutaneous (SC) injection
Dose:
- Prostate Cancer: 7.5 mg IM or SC once a month; 22.5 mg IM or SC every 3 months; 30 mg SC every 4 months; 45 mg SC every 6 months
- Endometriosis: 11.25 mg IM every 3 months for up to 12 months
- Uterine Fibroids: 11.25 mg IM every 3 months for up to 3 months
- Central Precocious Puberty: Dosing based on body weight; administered IM or SC once a month, every 3 months, or every 6 months
Mechanism of action: Leuprolide acetate is a synthetic GnRH agonist that initially stimulates the pituitary gland to release increased amounts of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This leads to a temporary surge in sex hormone levels. With continued administration, leuprolide desensitizes the GnRH receptors, resulting in a significant decrease in LH and FSH secretion, thereby suppressing the production of sex hormones such as testosterone in men and estrogen in women. This suppression is beneficial in conditions like prostate cancer, endometriosis, uterine fibroids, and central precocious puberty.
Drug usage cases:
- Prostate Cancer
- Endometriosis
- Uterine Fibroids
- Central Precocious Puberty
- Assisted Reproductive Technology (to control ovarian stimulation)
- Endometrial Thinning (prior to endometrial ablation)
Drug contraindications:
- Hypersensitivity to leuprolide acetate or any component of the formulation
- Pregnancy (Category X)
- Breastfeeding
- Undiagnosed abnormal vaginal bleeding
- Known or suspected pregnancy
Side effects:
- Hot flashes
- Injection site reactions (pain, swelling, redness)
- Headache
- Fatigue
- Joint pain
- Muscle pain
- Breast tenderness
- Vaginal dryness
- Decreased libido
- Depression
- Osteoporosis (with prolonged use)
- Elevated blood sugar levels
- Elevated cholesterol and triglyceride levels
- Elevated liver enzymes
- Injection site abscess (rare)
- Severe skin reactions (rare)
Warnings:
- Initial increase in testosterone levels may worsen symptoms of prostate cancer during the first few weeks of treatment
- Monitor bone mineral density due to potential bone loss with prolonged use
- Monitor blood glucose and lipid levels regularly
- Use with caution in patients with a history of depression or mood disorders
- May cause QT interval prolongation; monitor ECG in patients with cardiac risk factors
- Risk of anaphylaxis; observe patients for signs of hypersensitivity reactions
- Not recommended for use in pregnancy; may cause fetal harm
- Not recommended for use during breastfeeding
Use during pregnancy or breastfeeding: Leuprolide acetate is classified as a Category X medication, indicating that it may cause fetal harm when administered to a pregnant woman. Therefore, it is contraindicated during pregnancy. Women of childbearing potential should use effective contraception during treatment and for a period after discontinuation. Leuprolide is also contraindicated during breastfeeding, as it may be excreted in human milk and could harm a nursing infant.