Rexulti
23 June, 2023
Risperidone
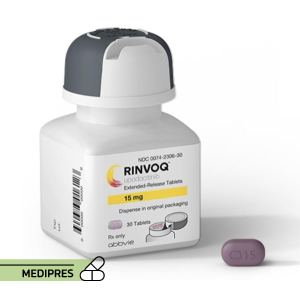
23 June, 2023Rinvoq
Generic name: upadacitinib
Drug class: Janus kinase (JAK) inhibitors
Dosage form: oral extended-release tablet, oral solution
Root of administration: oral
Dose:
- Rheumatoid arthritis: 15 mg once daily
- Psoriatic arthritis: 15 mg once daily
- Atopic dermatitis: 15 mg once daily
- Ulcerative colitis: 30 mg once daily for 8 weeks, then 15 mg once daily
- Crohn’s disease: 30 mg once daily for 12 weeks, then 15 mg once daily
- Ankylosing spondylitis: 15 mg once daily
- Non-radiographic axial spondyloarthritis: 15 mg once daily
- Giant cell arteritis: 30 mg once daily
Mechanism of action: Upadacitinib is a Janus kinase (JAK) inhibitor that reduces inflammation by blocking the activity of JAK enzymes, which are involved in the signaling pathways that lead to immune system activation and inflammation.
Drug usage cases:
- Moderately to severely active rheumatoid arthritis in adults who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers
- Active psoriatic arthritis in adults who have had an inadequate response or intolerance to one or more TNF blockers
- Active ankylosing spondylitis in adults who have had an inadequate response or intolerance to one or more TNF blockers
- Active non-radiographic axial spondyloarthritis with objective signs of inflammation in adults who have had an inadequate response or intolerance to TNF blocker therapy
- Refractory, moderate to severe atopic dermatitis in adults and pediatric patients 12 years of age and older whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable
- Moderately to severely active ulcerative colitis in adults who have had an inadequate response or intolerance to one or more TNF blockers
- Moderately to severely active Crohn’s disease in adults who have had an inadequate response or intolerance to one or more TNF blockers
- Active giant cell arteritis in adults
Drug contraindications:
- Active infections, including tuberculosis
- Known hypersensitivity to upadacitinib or any component of the formulation
- Severe hepatic impairment
- Concurrent use with other Janus kinase inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or potent immunosuppressants such as azathioprine and cyclosporine
Side effects:
- Upper respiratory tract infections (e.g., common cold, sinus infections)
- Nausea
- Cough
- Headache
- Fever
- Elevated blood pressure
- Elevated liver enzymes
- Elevated blood lipid levels
- Increased risk of serious infections, including tuberculosis
- Increased risk of certain cancers, including lymphoma and skin cancers
- Increased risk of major cardiovascular events, such as heart attack and stroke
- Increased risk of blood clots in veins and arteries
- Allergic reactions, including rash, difficulty breathing, and swelling of the face or throat
- Gastrointestinal perforations
- Changes in blood cell counts (e.g., low red or white blood cell counts)
Warnings:
- Serious infections: Increased risk of serious bacterial, fungal, viral, and opportunistic infections leading to hospitalization or death, including tuberculosis. Test for latent tuberculosis before and during therapy; treat latent tuberculosis prior to use. Monitor all patients for active tuberculosis during treatment, even those with initial negative latent tuberculosis tests.
- Cardiovascular events: Increased risk of major cardiovascular events, such as heart attack, stroke, and death, particularly in patients 50 years and older with at least one cardiovascular risk factor.
- Cancers: Increased risk of certain cancers, including lymphoma and skin cancers. Regular skin examinations are recommended during treatment.
- Blood clots: Increased risk of blood clots in veins and arteries, which can be life-threatening and cause death. This risk is higher in patients 50 years and older with at least one cardiovascular risk factor.
- Gastrointestinal perforations: Risk of tears in the stomach or intestines, especially in patients taking nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids. Seek immediate medical attention if experiencing stomach-area pain, fever, chills, nausea, or vomiting.
- Laboratory test abnormalities: Changes in certain laboratory tests, such as blood cell counts and liver enzymes, may occur. Regular monitoring is recommended during treatment.
Use during pregnancy or breastfeeding: The safety of upadacitinib during pregnancy and breastfeeding has not been established. It is recommended that women who are pregnant or breastfeeding avoid using upadacitinib. If you are pregnant, planning to become pregnant, or breastfeeding, consult your healthcare provider before starting treatment with upadacitinib.