Betaxolol
23 June, 2023
Biaxin Filmtab
23 June, 2023Bexarotene
Category: B
Description
Generic name:
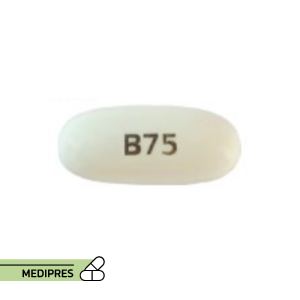
Bexarotene
Drug class:
Retinoid X receptor agonist (rexinoid)
Dosage form:
- Oral capsules: 75 mg
- Topical gel: 1 %, 3 %
Root of administration:
Oral, topical
Dose:
- Oral: 150–600 mg/m² once daily with food (typical starting dose 300 mg/m²/day)
- Topical: apply a thin film to affected areas once daily; adjust duration per response
- Varies by indication; consult label
Mechanism of action:
Bexarotene selectively binds and activates retinoid X receptors (RXR), modulating gene transcription to inhibit cell proliferation, and induce differentiation and apoptosis in malignant cells.
Drug usage cases:
- Cutaneous T-cell lymphoma (CTCL) refractory to at least one prior systemic therapy
- Mycosis fungoides
- Sézary syndrome
- Off-label: treatment of other cutaneous lymphomas and Kaposi’s sarcoma
Drug contra indications:
- Hypersensitivity to bexarotene or any component of the formulation
- Pregnancy (Category X)
- Severe hepatic impairment
- Concurrent vitamin A supplementation
- Pancreatitis or uncontrolled hyperlipidemia
Side effects:
- Hyperlipidemia (hypertriglyceridemia, hypercholesterolemia)
- Hypothyroidism
- Hepatotoxicity (elevated liver enzymes)
- Neutropenia, leukopenia, anemia, thrombocytopenia
- Headache, dizziness
- Fatigue
- Dry skin, pruritus, rash
- Mucocutaneous events: mucositis, cheilitis
- Pancreatitis
- Hyperglycemia
- Visual disturbances (blurred vision)
- Dyspepsia, nausea
- Photosensitivity
- Topical: erythema, burning, stinging, irritation, dryness
Warnings:
- Teratogenic: Category X—avoid in pregnancy; enforce contraception
- Monitor lipid profile and initiate lipid-lowering therapy if needed
- Monitor thyroid function and supplement thyroid hormone as indicated
- Monitor liver function tests regularly
- Risk of pancreatitis with severe hypertriglyceridemia
- Neutropenia: monitor blood counts
- Photosensitivity: counsel sun protection
- Use caution in patients with renal impairment
- Potential CYP3A4 interactions
Use during pregnancy or breastfeeding:
Pregnancy: Bexarotene is contraindicated (Category X). It is teratogenic, embryotoxic, and fetotoxic. Women of childbearing potential must use two effective forms of contraception one month prior to, during, and for two years after treatment. Men should use condoms during and for one month after treatment. Breastfeeding: discontinue during therapy and do not resume until at least two months after the last dose due to potential for serious adverse reactions in nursing infants.