AVSOLA (Infliximab-axxq Intravenous)
23 June, 2023
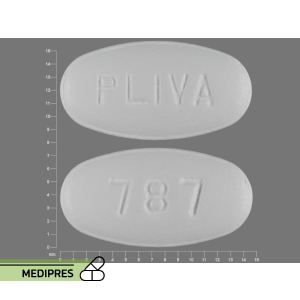
Baclofen
23 June, 2023Azithromycin (Intravenous)
Generic name:
Azithromycin
Drug class:
Macrolide antibiotic
Dosage form:
Intravenous infusion solution
Route of administration:
Intravenous (IV)
Dose:
Typical adult dose: 500 mg IV once daily; for some infections a loading dose of 500 mg on day 1 followed by 250 mg once daily on days 2–5. Dose ranges: 250–500 mg IV daily; infusion over at least 1 hour. Pediatric dose: loading dose 10 mg/kg IV once, then maintenance 5 mg/kg IV once daily. Adjustments vary by indication; consult label.
Mechanism of action:
Azithromycin binds to the 50S ribosomal subunit of susceptible bacteria, inhibiting translocation and protein synthesis.
Drug usage cases:
- Community-acquired pneumonia
- Acute bacterial sinusitis
- Skin and soft tissue infections
- Pharyngitis/tonsillitis
- Exacerbations of chronic obstructive pulmonary disease (COPD)
- Nongonococcal urethritis and cervicitis (Chlamydia trachomatis)
- Gonorrhea (Neisseria gonorrhoeae)
- Chancroid (Haemophilus ducreyi)
- Traveler’s diarrhea
- Prophylaxis of Mycobacterium avium complex in HIV patients
- Off-label: reduction of exacerbations in cystic fibrosis
Drug contraindications:
- Hypersensitivity to azithromycin, erythromycin, or any macrolide antibiotic
- History of cholestatic jaundice or hepatic dysfunction associated with prior macrolide use
Side effects:
- Gastrointestinal: diarrhea, nausea, abdominal pain, vomiting
- Injection site reactions: phlebitis, pain, inflammation
- Hepatotoxicity: elevated liver enzymes, hepatitis
- QT interval prolongation, torsades de pointes
- Headache, dizziness
- Hearing impairment (transient)
- Clostridioides difficile–associated diarrhea
- Hematologic: neutropenia, thrombocytopenia
- Hypersensitivity reactions: rash, urticaria, anaphylaxis
Warnings:
- QT prolongation: use with caution in patients with known risk factors
- Hepatic impairment: monitor liver function tests
- Clostridioides difficile–associated diarrhea risk; monitor for severe diarrhea
- Potential for resistant organisms; use appropriate culture data
- Infusion rate: administer over at least 1 hour to reduce phlebitis
- Caution in renal impairment; dosage adjustment may be necessary
Use during pregnancy or breastfeeding:
Pregnancy: Category B. Animal studies show no teratogenic effects; limited human data do not indicate increased risk of fetal harm. Use only if clearly needed. Breastfeeding: Azithromycin is excreted in breast milk; may cause diarrhea or candidiasis in nursing infants. Evaluate benefits versus potential risk; monitor infant for gastrointestinal symptoms.