Baclofen Injection
23 June, 2023
Baraclude
23 June, 2023Bafiertam
Generic name: monomethyl fumarate
Drug class: Selective immunosuppressants
Dosage form: Delayed-release capsules
Route of administration: Oral
Dose:
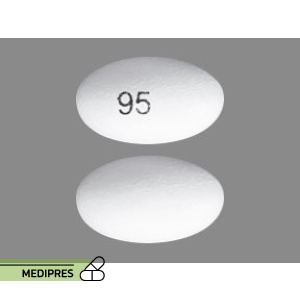
- Starting dose: 95 mg twice daily for 7 days
- Maintenance dose: 190 mg (administered as two 95 mg capsules) twice daily
- Temporary dosage reduction: 95 mg twice daily for individuals who do not tolerate the maintenance dose; within 4 weeks, resume the recommended dosage of 190 mg twice daily
Mechanism of action: Monomethyl fumarate is believed to exert its effects through activation of the Nrf2 pathway, leading to reduced oxidative cell stress and inflammation. This activation has demonstrated cytoprotective effects in human astrocytes and decreases the expression of vascular cell adhesion molecules, thereby reducing the adhesion and transendothelial migration of monocytes across an inflamed human blood–brain barrier.
Drug usage cases:
- Treatment of relapsing forms of multiple sclerosis in adults, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease
Drug contraindications:
- Known hypersensitivity to monomethyl fumarate, dimethyl fumarate, diroximel fumarate, or any of the excipients of Bafiertam
- Concurrent use with dimethyl fumarate or diroximel fumarate
Side effects:
- Flushing (sudden warmth, redness, or tingling feeling)
- Stomach pain, indigestion
- Nausea, vomiting
- Diarrhea
- Redness, itching, or rash
Warnings:
- Risk of serious brain infections, including progressive multifocal leukoencephalopathy (PML), which can lead to disability or death
- Potential for liver problems, including loss of appetite, upper right stomach pain, tiredness, dark urine, and jaundice (yellowing of the skin or eyes)
- Increased risk of serious infections, including herpes zoster (shingles) and other opportunistic infections
- Possible decrease in white blood cell count; regular blood tests are recommended to monitor this
Use during pregnancy or breastfeeding: The safety and effectiveness of Bafiertam during pregnancy and breastfeeding have not been established. It is not known if Bafiertam is safe and effective in children. Therefore, Bafiertam is not approved for use by anyone younger than 18 years old. If you are pregnant, plan to become pregnant, or are breastfeeding, consult your healthcare provider before starting treatment with Bafiertam.