Bafiertam
23 June, 2023
Baricitinib
23 June, 2023Baraclude
Category: B
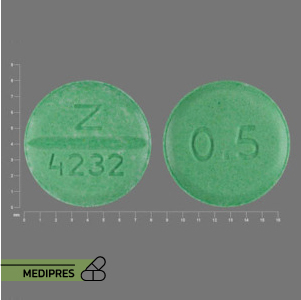
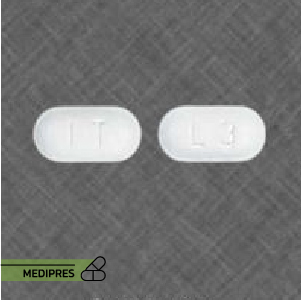
Description
Generic name: Entecavir
Drug class: Nucleoside analog antiviral
Dosage form: Tablets (0.5 mg and 1 mg), oral solution (0.05 mg/mL)
Route of administration: Oral
Dose:
- Adults with compensated liver disease: 0.5 mg once daily
- Adults with decompensated liver disease: 1 mg once daily
- Adults with lamivudine-resistant HBV: 1 mg once daily
- Children aged 2 to 18 years with compensated liver disease: Dose based on body weight
Mechanism of action: Entecavir is a guanosine nucleoside analog that inhibits HBV DNA polymerase by competing with the natural substrate deoxyguanosine triphosphate. This inhibition disrupts viral DNA synthesis, preventing HBV replication and reducing viral load.
Drug usage cases:
- Treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease
- Treatment of chronic HBV infection in adults with decompensated liver disease
- Treatment of chronic HBV infection in children aged 2 to 18 years with compensated liver disease
- Management of lamivudine-resistant HBV infection in adults
Drug contraindications:
- Hypersensitivity to entecavir or any component of the formulation
- Co-infection with HIV in patients not receiving antiretroviral therapy
Side effects:
- Common: Headache, fatigue, dizziness, nausea, vomiting, abdominal pain, diarrhea
- Serious: Lactic acidosis, hepatomegaly with steatosis, severe acute exacerbations of hepatitis B upon discontinuation
Warnings:
- Monitor for signs of lactic acidosis and hepatomegaly with steatosis
- Discontinuation may lead to severe acute exacerbations of hepatitis B; monitor liver function closely if stopping therapy
- Not recommended for HIV/HBV co-infected patients not receiving antiretroviral therapy
- Use caution in patients with renal impairment; dose adjustment may be necessary
Use during pregnancy or breastfeeding: Limited data; consult a healthcare provider before use during pregnancy or breastfeeding.