Gas-X
23 June, 2023
Gentamicin
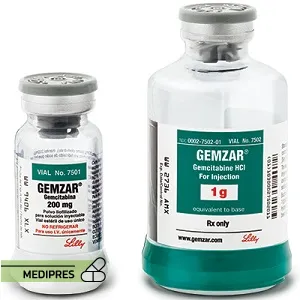
23 June, 2023Gemcitabine
Generic name: gemcitabine [ jem-SYE-ta-been ]
Brand names: Gemzar, Infugem
Drug class: Antimetabolites
Dosage Form: Injectable Solution
Route of Administration: Intravenous
Dose: intravenous powder for injection (1 g; 2 g; 200 mg), intravenous solution (10 mg/mL-NaCl 0.9%; 100 mg/mL; 38 mg/mL)
Mechanism of Action: Gemcitabine is metabolized into active nucleoside analogs that are incorporated into DNA, causing chain termination and apoptosis. It also inhibits ribonucleotide reductase, reducing the concentrations of deoxynucleotides available for DNA synthesis.
Drug Usage Cases: Gemcitabine is used for the treatment of various solid tumors, including pancreatic cancer, breast cancer, ovarian cancer, and non-small cell lung cancer.
Drug Contraindications: Contraindicated in patients with known hypersensitivity to gemcitabine.
Side Effects: Common side effects include nausea, vomiting, rash, and flu-like symptoms. Serious side effects can include myelosuppression, pulmonary toxicity, and hepatic insufficiency.
Warnings: Gemcitabine should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Regular monitoring of blood counts and liver function is recommended due to potential toxicity.
Use During Pregnancy or Breastfeeding: Gemcitabine is classified under FDA Pregnancy Category D. There is evidence of risk to the fetus based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. There is insufficient information on the excretion of gemcitabine in human milk.