Genebs
23 June, 2023
Geri-Tussin Expectorant
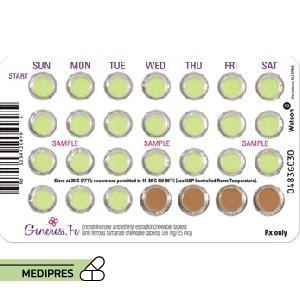
23 June, 2023Generess Fe
Generic name: Norethindrone and Ethinyl Estradiol Chewable Tablets, Ferrous Fumarate Chewable Tablets
Drug class: Contraceptives
Dosage form: Tablets
Root of administration: Oral
Dose: One tablet daily for 28 consecutive days, taken at the same time each day without water. The regimen includes 24 active tablets containing 0.8 mg norethindrone and 0.025 mg ethinyl estradiol, followed by 4 inactive tablets containing 75 mg ferrous fumarate.
Mechanism of action: Combination oral contraceptives primarily prevent pregnancy by suppressing ovulation. Additional mechanisms may include changes in cervical mucus that inhibit sperm penetration and alterations in the endometrium that reduce the likelihood of implantation.
Drug usage cases:
- Prevention of pregnancy in females of reproductive potential.
Drug contraindications:
- High risk of arterial or venous thrombotic diseases, including smokers over 35 years old.
- Undiagnosed abnormal uterine bleeding.
- Breast cancer or other estrogen or progestin-sensitive neoplasms.
- Liver tumors or liver disease.
- Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir.
Side effects:
- Nausea/vomiting.
- Headaches/migraines.
- Depression/mood complaints.
- Dysmenorrhea.
- Acne.
- Anxiety symptoms.
- Breast pain/tenderness.
- Increased weight.
Warnings:
- Cigarette smoking increases the risk of serious cardiovascular events from oral contraceptive use, especially in women over 35 years old.
- Discontinue if a thrombotic event occurs.
- Discontinue if jaundice occurs.
- Discontinue if significant changes in headaches occur.
- Discontinue at least 4 weeks before and through 2 weeks after major surgery associated with increased risk of thromboembolism.
- Not recommended for women with uncontrolled hypertension.
- Not recommended for women with uncontrolled dyslipidemia.
- Not recommended for women with uncontrolled diabetes.
- Not recommended for women with gallbladder disease.
- Not recommended for women with hypertriglyceridemia.
- Not recommended for women with pregnancy-related cholestasis.
- Not recommended for women with depression.
- Not recommended for women with BMI >35 kg/m².
Use during pregnancy or breastfeeding:
Discontinue if pregnancy occurs.
Not recommended for nursing mothers, as it can decrease milk production.