Genebs
23 June, 2023
Goserelin
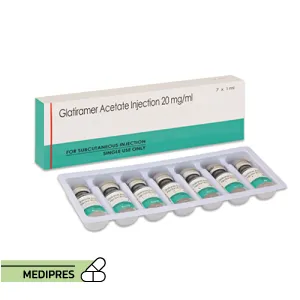
23 June, 2023Glatiramer
Generic name: glatiramer [ gla-TIR-a-mer-AS-e-tate ]
Drug Class: Immunomodulator
Dosage Form: Injectable Solution
Route of Administration: Subcutaneous
Dose:
- Injectable Solution: Commonly 20 mg/mL, administered daily
Mechanism of Action: Glatiramer acetate is thought to act by modifying immune processes that are believed to be responsible for the pathogenesis of multiple sclerosis (MS). It may enhance suppressor T cell activity and downregulate damaging inflammatory responses.
Drug Usage Cases: Glatiramer is used primarily in the treatment of relapsing forms of multiple sclerosis (MS) to decrease the frequency of relapses and delay the accumulation of physical disability.
Drug Contraindications: Contraindicated in patients with known hypersensitivity to glatiramer acetate or mannitol.
Side Effects: Common side effects include injection site reactions, flushing, chest pain, palpitations, anxiety, and dyspnea. These symptoms often occur shortly after injection and usually resolve on their own.
Warnings: Patients are advised to rotate injection sites to minimize the risk of lipoatrophy or skin necrosis at the injection site.
Use During Pregnancy or Breastfeeding: Glatiramer is classified under FDA Pregnancy Category B. Animal studies have not demonstrated a risk to the fetus, and there are no adequate and well-controlled studies in pregnant women. Glatiramer should be used during pregnancy only if clearly needed. There is insufficient information regarding the excretion of glatiramer in human milk.