Geodon
23 June, 2023
Glucagon
23 June, 2023Glipizide
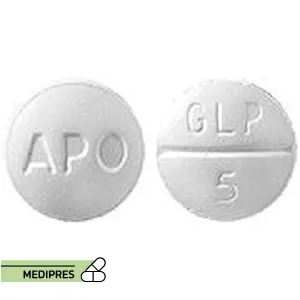
Generic name: glipizide [ GLIP-i-zide ]
Brand name: Glucotrol
Drug Class: Sulfonylurea
Dosage Form: Tablets (regular and extended-release)
Route of Administration: Oral
Dose:
- Regular Tablets: Typically starts at 5 mg, may be adjusted based on response
- Extended-Release Tablets: Starts at 2.5 mg to 5 mg, can go up to 20 mg daily
Mechanism of Action: Glipizide stimulates the release of insulin from the pancreatic beta cells. It also increases the sensitivity of peripheral tissues to insulin, which leads to improved glucose uptake and metabolism.
Drug Usage Cases: Glipizide is used primarily for the control of blood sugar levels in patients with type 2 diabetes mellitus as part of a diet and exercise program.
Drug Contraindications: Contraindicated in patients with type 1 diabetes, diabetic ketoacidosis, or those with a known hypersensitivity to sulfonylureas.
Side Effects: Common side effects include hypoglycemia, headache, nausea, and dizziness. Serious side effects can include severe hypoglycemia, hepatotoxicity, and hypersensitivity reactions.
Warnings: Patients should be aware of the symptoms of hypoglycemia and be prepared to manage them. Glipizide should be used with caution in patients with liver or kidney impairment due to the increased risk of hypoglycemia.
Use During Pregnancy or Breastfeeding: Glipizide is classified under FDA Pregnancy Category C. It is not recommended during pregnancy as it may cause hypoglycemia in the newborn. There is limited data on the excretion of glipizide in human milk; caution should be exercised when glipizide is administered to a nursing mother.