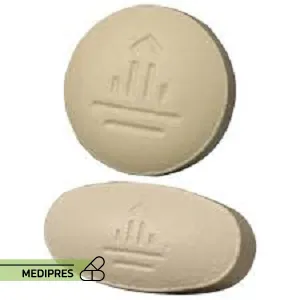
Janumet
23 June, 2023Jatamansi
23 June, 2023Jardiance
Generic name: empagliflozin [ EM-pa-gli-FLOE-zin ]
Drug class: SGLT2 inhibitors (Sodium-glucose co-transporter 2 inhibitors)
Dosage form: Tablet
Route of administration: Oral
Dose: Typically, 10 mg once daily, which may be increased to 25 mg once daily based on patient response and tolerability.
Mechanism of action: Jardiance works by inhibiting the SGLT2 protein in the proximal renal tubules, which reduces glucose reabsorption in the kidneys and increases glucose excretion in the urine. This helps to lower blood glucose levels in patients with type 2 diabetes.
Drug usage cases: Jardiance is used to improve glycemic control in adults with type 2 diabetes mellitus and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
Drug contra indications: Jardiance should not be used in patients with severe renal impairment, end-stage renal disease, or on dialysis. It is also contraindicated in patients with known hypersensitivity to empagliflozin or any component of the formulation.
Side effects: Common side effects include urinary tract infections, increased urination, and genital infections. Serious side effects may include:
- Ketoacidosis: Risk of diabetic ketoacidosis even with normal blood glucose levels.
- Acute kidney injury: Potential for worsening renal function.
- Hypotension: Risk of low blood pressure due to diuresis.
- Hypoglycemia: Particularly when used with insulin or sulfonylureas.
Warnings: Jardiance can increase the risk of ketoacidosis and should be discontinued in patients who develop symptoms of this condition. Renal function should be monitored before and during treatment. Patients should be advised to maintain adequate hydration to prevent hypotension and acute kidney injury.
Use during pregnancy or breastfeeding: Jardiance is not recommended during the second and third trimesters of pregnancy due to the potential for adverse effects on renal development in the fetus. It is unknown if empagliflozin is excreted in human milk, so caution should be exercised when administered to breastfeeding mothers.