Jencycla
23 June, 2023
Jublia
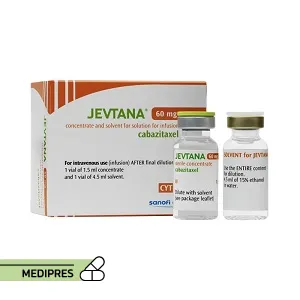
23 June, 2023Jevtana
Generic name: cabazitaxel [ ka-BAZ-i-TAX-el ]
Drug class: Antineoplastics (Taxanes)
Dosage form: Injection
Route of administration: Intravenous (IV)
Dose: Typically, 25 mg/m² administered as a 1-hour IV infusion every three weeks, in combination with prednisone.
Mechanism of action: Jevtana disrupts microtubule function by promoting tubulin assembly and inhibiting disassembly, leading to cell cycle arrest and apoptosis in cancer cells. This mechanism targets rapidly dividing tumor cells, particularly in prostate cancer.
Drug usage cases: Jevtana is used to treat hormone-refractory metastatic prostate cancer in patients previously treated with a docetaxel-containing regimen.
Drug contra indications: Jevtana should not be used in patients with severe hypersensitivity to cabazitaxel, other taxanes, or polysorbate 80. It is contraindicated in patients with neutrophil counts below 1,500/mm³ or severe hepatic impairment.
Side effects: Common side effects include neutropenia, anemia, diarrhea, and fatigue. Serious side effects may include:
- Neutropenia: Risk of severe and febrile neutropenia.
- Hypersensitivity reactions: Including anaphylaxis.
- Gastrointestinal toxicity: Severe diarrhea, nausea, and vomiting.
- Hepatotoxicity: Potential for liver enzyme elevation and liver damage.
Warnings: Jevtana can cause severe neutropenia, requiring frequent blood count monitoring and the use of growth factor support if necessary. Hypersensitivity reactions necessitate premedication with antihistamines and corticosteroids. Gastrointestinal toxicity management may require supportive care and dose adjustments.
Use during pregnancy or breastfeeding: Jevtana can cause fetal harm when administered to pregnant women. It is not recommended during pregnancy. Women of childbearing potential should use effective contraception during treatment. It is unknown whether cabazitaxel is excreted in human milk, so breastfeeding should be discontinued during treatment.