Kalydeco
23 June, 2023
Keveyis
23 June, 2023Ketek
Generic name: telithromycin
Drug class: ketolide antibiotic
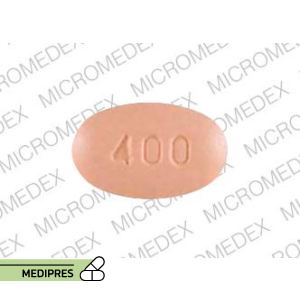
Dosage form: tablets (400 mg)
Root of administration: oral
Dose: 800 mg once daily for 10 days (community-acquired bacterial pneumonia); 400 mg twice daily for 7–10 days (acute bacterial exacerbations of chronic bronchitis); 400 mg twice daily for 5–10 days (pharyngitis/tonsillitis)
Mechanism of action: binds to the 50S bacterial ribosomal subunit, inhibiting protein synthesis by blocking peptidyl transferase and translocation; retains activity against some macrolide-resistant strains
Drug usage cases:
- Community-acquired bacterial pneumonia
- Acute bacterial exacerbations of chronic bronchitis
- Pharyngitis and tonsillitis
- Off-label: skin and soft tissue infections; sinusitis; atypical mycobacterial infections (varies by indication; consult label)
Drug contra indications:
- Hypersensitivity to telithromycin, ketolides, or macrolides
- Myasthenia gravis or history of myasthenia gravis
- Severe hepatic impairment
- Known QT prolongation or history of torsades de pointes
- Concomitant use with CYP3A4 substrates: cisapride, pimozide, ergot alkaloids, lovastatin, simvastatin
Side effects:
- Gastrointestinal: diarrhea, nausea, vomiting, abdominal pain, dyspepsia
- Hepatic: elevated transaminases, hepatitis, cholestatic jaundice, hepatic failure
- Cardiac: QT interval prolongation, arrhythmias
- Central nervous system: headache, dizziness, insomnia, visual disturbances (blurred vision, difficulty focusing)
- Auditory: tinnitus
- Dermatologic: rash, Stevens–Johnson syndrome, toxic epidermal necrolysis
- Hematologic: eosinophilia, leukopenia
- Neuromuscular: exacerbation of myasthenia gravis
- Other: taste perversion, fatigue
Warnings:
- Severe hepatotoxicity: monitor liver function; discontinue if signs of liver injury occur
- QT prolongation and torsades de pointes: use with caution in patients with risk factors
- Contraindicated in myasthenia gravis due to risk of exacerbation
- Numerous CYP3A4 interactions: review all concomitant medications
- Use caution in hepatic impairment
- Immune thrombocytopenia reported: monitor if bleeding/bruising occurs
- Visual disturbances reported: advise patients
- Clostridioides difficile–associated diarrhea may occur
Use during pregnancy or breastfeeding:
Pregnancy: Category C. Animal studies have shown adverse fetal effects; no adequate human data. Use only if benefits justify potential fetal risk.
Breastfeeding: Excreted in human milk; potential for serious adverse reactions in nursing infants. Discontinue breastfeeding during treatment.