Kimmtrak
23 June, 2023
Klor-Con M
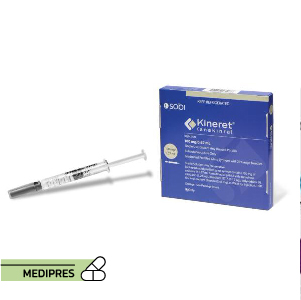
23 June, 2023Kineret
Generic name: Anakinra
Drug classes: Antirheumatics, Interleukin inhibitors
Dosage form: Subcutaneous Injection
Root of administration: Subcutaneous
Dose: 100, 200 mg
Mechanism of action: Kineret works to reduce the body’s immune response and inflammation by blocking the IL-1 receptor as it is an interleukin-1 (IL-1) receptor antagonist.
Drug usage cases: Kineret is a FDA approved medicine used to treat moderate to severe rheumatoid arthritis in adults, neonatal onset multisystem inflammatory disease (NOMID) in newborn babies, and deficiency of interleukin-1 receptor antagonist (DIRA) in adults and children. Kineret also has emergency use authorization (EUA) for the treatment of COVID-19 in specific hospitalized patients.
Drug contra indications: Do not give Kineret to anyone under 18 years old without medical advice. You should not use Kineret if you are allergic to anakinra or if you have:
an active infection; or an allergy to any medicine that contains E. coli bacteria proteins.
To make sure Kineret is safe for you, tell your doctor if you have:
kidney disease, an active or chronic infection, fever, chills, or open sores on your skin, a weak immune system (caused by disease or by using certain medicines): asthma, tuberculosis; or if you are scheduled to receive a vaccine. Do not give this medicine to a child without medical advice. Kineret is not approved to treat rheumatoid arthritis in anyone younger than 18 years old.
Side effects: Get emergency medical help if you have signs of an allergic reaction to Kineret: hives, sweating, severe itching; wheezing, difficult breathing; fast or pounding heartbeats; dizziness, fainting; swelling of your face, lips, tongue, or throat. If you are using Kineret for DIRA, you may have an increased risk of allergic reactions, especially in the first weeks of treatment. Call your doctor at once if you have:
fever or chills, low white blood cell counts – mouth sores, skin sores, sore throat; or signs of tuberculosis – fever, cough, night sweats, loss of appetite, weight loss, and feeling very tired.
Warnings: The US Food and Drug Administration (FDA) has authorized emergency use of Kineret for the treatment of COVID-19 in hospitalized adults who tested positive for COVID-19 with pneumonia requiring supplemental oxygen, who are at risk of having severe respiratory failure, and are likely to have an increased risk of worsening of the disease (measured by abnormal blood levels of a certain protein). You should not use Kineret if you are allergic to medicines that contain E. coli bacteria proteins, or if you have an active infection. Tell doctor if you have: latex allergy, a weak immune system, an active or chronic infection, or signs of infection such as fever, chills, or open sores on your skin. Kineret can lower blood cells that help your body fight infections. Your blood may need to be tested often. Avoid being near people who are sick or have infections. Stop using this medicine and call your doctor right away if you have signs of infection such as: fever, chills, flu symptoms, mouth sores, weight loss, or feeling tired or short of breath. You may have a higher risk of infection if you are also using adalimumab (Humira), certolizumab (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade), adalimumab (Humira), cancer medicines, steroids, or medicines to prevent organ transplant rejection.
Use during pregnancy or breastfeeding: Tell your doctor if you are pregnant or plan to become pregnant. Having rheumatoid arthritis or CAPS during pregnancy may increase the risk of premature birth or low birth weight. The benefit of treating rheumatoid arthritis or CAPS may outweigh any risks to the baby. It may not be safe to breastfeed while using this medicine. Ask your doctor about any risk.