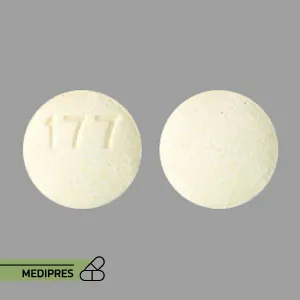
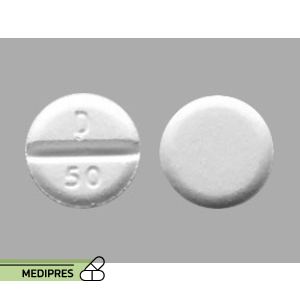
Xpovio
11 February, 2024
Kyprolis
19 May, 2024Kuvan
Drug name: Kuvan
Drug class: Phenylalanine hydroxylase activators
Dosage form: Tablet, Powder for oral solution
Route of administration: Oral
Dose: Typically, 10 mg/kg to 20 mg/kg once daily.
Mechanism of action: Kuvan is a synthetic form of the naturally occurring cofactor tetrahydrobiopterin (BH4), which enhances the activity of phenylalanine hydroxylase (PAH) to reduce blood phenylalanine levels in patients with phenylketonuria (PKU).
Drug usage cases: Kuvan is used to lower blood phenylalanine levels in patients with hyperphenylalaninemia due to tetrahydrobiopterin-responsive phenylketonuria (PKU).
Drug contra indications: Kuvan should not be used in patients with known hypersensitivity to sapropterin dihydrochloride or any component of the formulation.
Side effects: Common side effects include headache, rhinorrhea, and gastrointestinal disturbances. Serious side effects may include:
- Allergic reactions: Risk of hypersensitivity reactions.
- Hypophosphatemia: Low phosphate levels in the blood.
- Hyperactivity: Increased activity levels, particularly in children.
- Gastrointestinal bleeding: Risk of bleeding in the digestive tract.
Warnings: Kuvan can cause allergic reactions and patients should be monitored for signs of hypersensitivity. Blood phosphate levels should be monitored due to the risk of hypophosphatemia. Patients should be advised to maintain dietary restrictions to manage phenylalanine levels effectively.
Use during pregnancy or breastfeeding: The safety of Kuvan during pregnancy is not well established, and it should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is unknown whether sapropterin is excreted in human milk, so caution should be exercised when administered to breastfeeding mothers.