LYMEPAK
23 June, 2023
Magnesium lactate
23 June, 2023Lysteda
Category: L
Description
Generic name:
Tranexamic acid
Drug class:
Antifibrinolytic agent
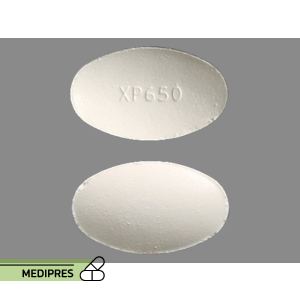
Dosage form:
Oral tablet: 650 mg
Root of administration:
Oral
Dose:
- 1300 mg (two 650 mg tablets) orally three times daily during menstrual bleeding, up to 5 days
- Alternative regimens: 1 g orally three times daily or 1–1.5 g every 8 hours for up to 4–5 days
- Varies by indication; consult label
Mechanism of action:
Tranexamic acid is a synthetic lysine analog that reversibly binds to plasminogen, blocking its interaction with fibrin and inhibiting fibrinolysis, thereby stabilizing formed clots.
Drug usage cases:
- Heavy menstrual bleeding (menorrhagia) – FDA-approved
- Epistaxis (nosebleeds) – off-label
- Hematuria – off-label
- Perioperative bleeding prophylaxis (dental, cardiac, orthopedic surgeries) – off-label
- Trauma-related hemorrhage – off-label
- Bleeding in hemophilia patients undergoing dental procedures – off-label
Drug contra indications:
- Active intravascular clotting (e.g., disseminated intravascular coagulation) unless fibrinolysis is the predominant problem
- History of thrombosis or thromboembolic disease (e.g., DVT, PE) unless benefits outweigh risks
- Subarachnoid hemorrhage
- Hypersensitivity to tranexamic acid or any excipients
- Acquired defective color vision
Side effects:
- Nausea, vomiting, diarrhea
- Abdominal pain
- Headache, dizziness
- Fatigue, malaise
- Muscle cramps, myalgia, back pain
- Joint pain (arthralgia)
- Hypotension (especially with rapid IV administration)
- Thromboembolic events (deep vein thrombosis, pulmonary embolism)
- Visual disturbances, changes in color vision
- Seizures (rare, higher risk at high doses)
- Hypersensitivity reactions (rash, urticaria, anaphylaxis)
- Renal cortical necrosis (rare, with high doses or compromised renal function)
Warnings:
- Risk of thromboembolic events; use with caution in patients with history of clotting disorders
- Monitor renal function; dose adjustment required in renal impairment
- Caution in patients using hormonal contraceptives or estrogens (additive thrombosis risk)
- Seizure risk increased at high doses or in predisposed patients
- Stop therapy if signs of thrombosis occur (e.g., leg pain, swelling, dyspnea)
- Avoid rapid IV administration to prevent hypotension
- Use caution in patients with cardiovascular disease, pulmonary disease, or renal impairment
- Avoid in patients with acquired defective color vision
- Hypersensitivity: discontinue if severe allergic reaction occurs
Use during pregnancy or breastfeeding:
Pregnancy category B. Animal studies have not shown fetal harm, but adequate human studies are lacking. Use only if potential benefit justifies risk to fetus. Tranexamic acid crosses the placenta.
In breastfeeding, tranexamic acid is excreted in human milk in low concentrations. Monitor infants for adverse effects; weigh benefits of therapy against potential risks to the nursing infant.