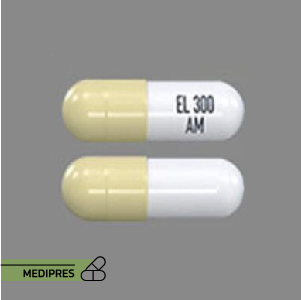
Ongentys
23 June, 2023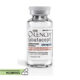
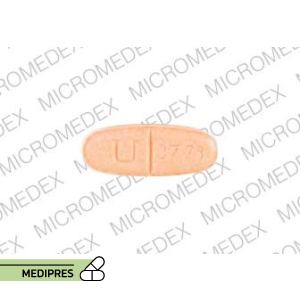
Orencia
23 June, 2023Opdivo
Category: O
Description
Generic name:
Nivolumab
Drug class:
Programmed death-1 (PD-1) immune checkpoint inhibitor (monoclonal antibody)
Dosage form:
- Concentrate for intravenous infusion: 10 mg/mL in 40 mg/4 mL and 100 mg/10 mL vials
- Prefilled solution for infusion: 240 mg/8 mL and 480 mg/16 mL
Route of administration:
Intravenous infusion
Dose:
Varies by indication; consult label.
- 3 mg/kg every 2 weeks
- 240 mg every 2 weeks
- 480 mg every 4 weeks
- Combination with ipilimumab: 1 mg/kg nivolumab + 3 mg/kg ipilimumab every 3 weeks for 4 doses, then maintenance nivolumab
Mechanism of action:
Nivolumab is a fully human IgG4 monoclonal antibody that binds to PD-1 receptors on activated T cells, blocking interaction with PD-L1/PD-L2 ligands and restoring anti-tumor T-cell activity.
Drug usage cases:
- Unresectable or metastatic melanoma
- Adjuvant treatment of melanoma with lymph node involvement
- Non–small cell lung cancer (NSCLC), metastatic or recurrent
- NSCLC, adjuvant therapy following resection and chemotherapy
- Renal cell carcinoma, advanced
- Classical Hodgkin lymphoma, relapsed or refractory
- Head and neck squamous cell carcinoma, recurrent or metastatic
- Urothelial carcinoma, locally advanced or metastatic
- Microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer
- Hepatocellular carcinoma, previously treated
- Esophageal or gastroesophageal junction carcinoma, advanced
- Gastric cancer, advanced
- Endometrial carcinoma, MSI-H or dMMR
- Biliary tract carcinoma, advanced
- Cervical cancer, recurrent or metastatic
- Off-label: Varies by indication; consult treatment guidelines
Drug contraindications:
- History of severe hypersensitivity to nivolumab or any excipient
Side effects:
- Immune-mediated pneumonitis
- Immune-mediated colitis
- Immune-mediated hepatitis
- Immune-mediated nephritis and renal dysfunction
- Immune-mediated endocrinopathies (hypophysitis, thyroid disorders, adrenal insufficiency)
- Dermatologic reactions (rash, pruritus)
- Infusion-related reactions
- Fatigue
- Diarrhea
- Nausea and vomiting
- Decreased appetite
- Cough and dyspnea
- Musculoskeletal pain (arthralgia, myalgia)
- Fever
- Headache
- Electrolyte abnormalities (hypocalcemia, hyponatremia)
Warnings:
- Immune-mediated adverse reactions can be severe or fatal; monitor and manage promptly with corticosteroids
- Hold or discontinue for Grade 2 or greater immune-mediated events per guidelines
- Infusion-related reactions: monitor during and after infusion; manage with supportive care or corticosteroids
- Embryo-fetal toxicity: advise effective contraception during and for 5 months after treatment
- Avoid live vaccines during and after therapy until immune recovery
- Caution in patients with preexisting autoimmune disorders
- Monitor liver, renal, thyroid, adrenal function, and glycemic control
Use during pregnancy or breastfeeding:
Nivolumab can cause fetal harm. Pregnancy testing is recommended prior to initiation. Women of childbearing potential should use effective contraception during therapy and for at least 5 months after the last dose. It is unknown if nivolumab is excreted in human milk; due to potential for serious adverse reactions in nursing infants, discontinue breastfeeding during treatment and for 5 months after the last dose.