Quartette
23 June, 2023
Quelicin
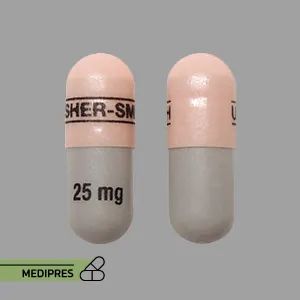
23 June, 2023Qudexy XR
Generic name: topiramate [ toe-PIR-a-mate ]
Drug class: Carbonic anhydrase inhibitor anticonvulsants
Brand name: Qudexy XR
Dosage forms: Extended-release capsules.
Route of administration: Oral.
Dose: Epilepsy (Seizures): Initial dose: 25 mg to 50 mg once daily. Maintenance dose: Typically 100 mg to 400 mg per day, divided into two doses. Migraine Prevention: Initial dose: 25 mg once daily. Maintenance dose: 100 mg to 200 mg per day, divided into two doses.
Mechanism of action: Topiramate’s exact mechanism of action is not fully understood, but it is believed to involve multiple pathways. It inhibits excitatory neurotransmitter activity, enhances GABAergic neurotransmission, and stabilizes neuronal membranes. This combined effect helps to reduce the frequency of seizures and prevent migraines.
Drug usage cases: Treatment of epilepsy (partial-onset seizures and primary generalized tonic-clonic seizures). Prevention of migraines.
Drug contraindications: Known hypersensitivity to topiramate or any component of the formulation. Use in patients with a history of metabolic acidosis or severe renal impairment. Caution in patients with a history of kidney stones, glaucoma, or liver disease.
Side effects: Common: Drowsiness, dizziness, weight loss, tingling in the limbs, difficulty concentrating. Serious: Severe allergic reactions, metabolic acidosis, kidney stones, eye problems (e.g., acute myopia, glaucoma), suicidal thoughts or behavior. Rare: Serious skin reactions, such as Stevens-Johnson syndrome.
Warnings: Monitor for signs of metabolic acidosis and kidney stones. Regular eye exams may be necessary to check for potential eye problems. Be alert to changes in mood or behavior, including suicidal thoughts. Use with caution in patients with a history of kidney stones or other significant health conditions. Adjust dosage gradually to minimize the risk of adverse effects.
Use during pregnancy or breastfeeding: Use during pregnancy only if the potential benefit justifies the potential risk to the fetus. Topiramate can cause harm to the developing fetus, including an increased risk of congenital malformations. It is unknown if topiramate is excreted in breast milk, so consult your doctor before using this medication while breastfeeding.