Rivaroxaban
23 June, 2023
Ropinirole
23 June, 2023Rivastigmine
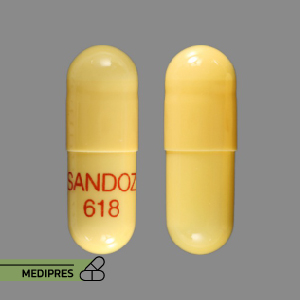
Generic name: rivastigmine (oral) [ ri-va-STIG-meen ]
Brand name: Exelon
Dosage form: oral capsule (1.5 mg; 3 mg; 4.5 mg; 6 mg)
Drug Class: Cholinesterase inhibitors
Route of administration: Oral, transdermal.
Dose: Alzheimer’s Disease: Initial oral dose: 1.5 mg twice daily, may increase to a maximum of 6 mg twice daily based on tolerability. Transdermal patch: 4.6 mg/24 hours as an initial dose, can be titrated up to 13.3 mg/24 hours based on patient response and tolerance.
Mechanism of action: Rivastigmine inhibits acetylcholinesterase and butyrylcholinesterase, enzymes responsible for breaking down acetylcholine in the brain. By increasing the levels of acetylcholine, rivastigmine helps improve cognitive function in patients with Alzheimer’s disease and Parkinson’s disease-related dementia.
Drug usage cases: Treatment of mild to moderate dementia associated with Alzheimer’s disease. Treatment of mild to moderate dementia associated with Parkinson’s disease.
Drug contraindications: Known hypersensitivity to rivastigmine, other carbamate derivatives, or any component of the formulation. History of application-site reactions with the transdermal patch that may suggest allergic contact dermatitis.
Side effects: Common: Nausea, vomiting, diarrhea, loss of appetite, dizziness. Serious: Severe gastrointestinal effects (vomiting, dehydration), severe skin reactions, bradycardia, syncope. Rare: Severe allergic reactions, exacerbation of asthma, or obstructive pulmonary disease.
Warnings: Monitor for gastrointestinal adverse reactions, especially during dose escalation. Use caution in patients with a history of peptic ulcer disease, asthma, or obstructive pulmonary disease. Be aware of the potential for allergic contact dermatitis with the transdermal patch. Patients and caregivers should be instructed on the proper use and disposal of the transdermal patch.
Use during pregnancy or breastfeeding: The safety of rivastigmine during pregnancy has not been established; use only if the potential benefit justifies the potential risk to the fetus. It is not known whether rivastigmine is excreted in human milk; therefore, caution should be exercised if administered to breastfeeding mothers.