Sapropterin Oral Solution
23 June, 2023
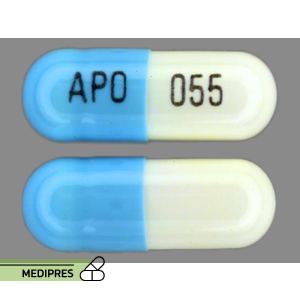
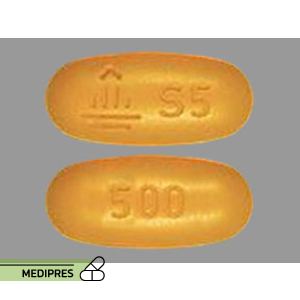
Selegiline Tablets
23 June, 2023Selegiline
Generic name: Selegiline
Drug class: Monoamine oxidase inhibitor (MAOI); Catecholaminergic activity enhancer; Norepinephrine releasing agent; Antiparkinsonian; Antidepressant
Dosage form: Oral tablets and capsules (5 mg); Orally disintegrating tablets (1.25 mg); Transdermal patches (6, 9, and 12 mg/24 hours)
Route of administration: Oral (tablets, capsules, disintegrating tablets); Transdermal (patches)
Dose:
- Parkinson’s disease: Oral tablets/capsules: 5 mg twice daily (10 mg/day); Orally disintegrating tablets: 1.25 mg once daily, may increase to 2.5 mg once daily after 6 weeks; Transdermal patches: 6 mg/24 hours once daily, may increase to 9 mg/24 hours or 12 mg/24 hours after 2 weeks
- Depression: Transdermal patches: 6 mg/24 hours once daily, may increase to 9 mg/24 hours or 12 mg/24 hours after 2 weeks
Mechanism of action: Selegiline is an irreversible monoamine oxidase B (MAO-B) inhibitor at lower doses, increasing brain levels of dopamine. At higher doses, it also inhibits MAO-A, increasing serotonin and norepinephrine levels. Additionally, selegiline enhances the release of norepinephrine and dopamine in the brain through its catecholaminergic activity enhancer properties.
Drug usage cases:
- Treatment of Parkinson’s disease
- Adjunct therapy with levodopa in Parkinson’s disease
- Treatment of major depressive disorder
Drug contraindications:
- Hypersensitivity to selegiline
- Concurrent use with meperidine (DEMEROL) and other opioids
- Concurrent use with other monoamine oxidase inhibitors (MAOIs) such as linezolid, phenelzine, and tranylcypromine
- Concurrent use with dextromethorphan, St. John’s wort, cyclobenzaprine, pentazocine, propoxyphene, or carbamazepine
Side effects:
- Nausea
- Hallucinations
- Confusion
- Depression
- Loss of balance
- Insomnia
- Increased involuntary movements
- Agitation
- Slow or irregular heart rate
- Delusions
- Hypertension
- New or increased angina pectoris
- Syncope
- Orthostatic hypotension
- Atrial fibrillation
- Other cardiac arrhythmias
- Application-site reactions (for transdermal patch)
- Dry mouth
- Dizziness
- Nervousness
- Abnormal dreams
Warnings:
- Risk of serotonin syndrome when combined with serotonergic agents
- Risk of hypertensive crisis with tyramine-rich foods, especially at higher doses
- Potential for severe CNS toxicity when combined with tricyclic antidepressants and non-selective MAOIs
- Use caution in patients with cardiovascular conditions due to potential arrhythmias
- Monitor for unusual changes in behavior, such as compulsive urges
- Gradual dose reduction recommended to discontinue therapy
- Monitor for signs of orthostatic hypotension
Use during pregnancy or breastfeeding:
Selegiline is classified as a Category C drug during pregnancy, indicating that risk to the fetus cannot be ruled out. It should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. The safety of selegiline during breastfeeding has not been established; therefore, it should be used with caution in nursing mothers, and the decision to discontinue breastfeeding or the drug should be made based on the importance of the drug to the mother. Consult a healthcare provider for personalized advice.