Simponi Aria
23 June, 2023
Slo-Niacin
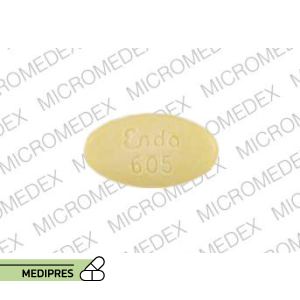
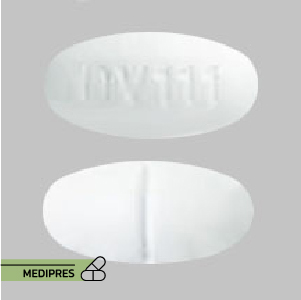
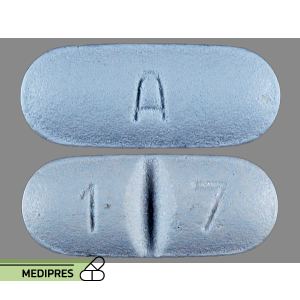
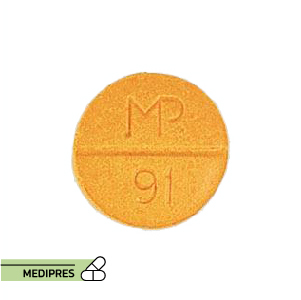
23 June, 2023Sinemet 25-100
Generic name: Carbidopa and Levodopa
Drug class: Dopaminergic antiparkinsonism agents
Dosage form: Tablet
Route of administration: Oral
Dose:
- Initial: One tablet (25 mg carbidopa/100 mg levodopa) three times daily
- Maintenance: Adjusted based on therapeutic response, typically up to eight tablets per day
- Maximum: Eight tablets (25 mg carbidopa/100 mg levodopa) per day
Mechanism of action: Carbidopa inhibits the peripheral decarboxylation of levodopa to dopamine, increasing levodopa availability to the brain and reducing peripheral side effects.
Drug usage cases:
- Treatment of Parkinson’s disease
- Management of parkinsonism symptoms
Drug contraindications:
- Hypersensitivity to carbidopa, levodopa, or any component of the formulation
- Use of non-selective monoamine oxidase inhibitors (MAOIs) within the last 14 days
- Angle-closure glaucoma
- Suspicion of undiagnosed skin lesions or a history of melanoma
- Non-traumatic, uncorrected orthostatic hypotension
- History of neuroleptic malignant syndrome (NMS) or non-traumatic rhabdomyolysis
Side effects:
- Nausea and vomiting
- Dizziness
- Orthostatic hypotension
- Involuntary movements (dyskinesias)
- Hallucinations
- Confusion
- Depression
- Darkening of urine, saliva, or sweat
- Unusual body movements
- Sleep disturbances
Warnings:
- Monitor for signs of orthostatic hypotension, especially during dose initiation and titration
- Exercise caution in patients with a history of cardiovascular disease
- Regularly assess for the development of involuntary movements (dyskinesias)
- Be vigilant for psychiatric effects, including hallucinations and depression
- Discontinue if signs of neuroleptic malignant syndrome (NMS) occur
- Monitor for signs of melanoma in patients with a history of skin lesions
- Use cautiously in patients with a history of peptic ulcer disease or gastrointestinal bleeding
- Adjust dosage in patients with hepatic or renal impairment
- Monitor for signs of hemolytic anemia, leukopenia, or thrombocytopenia
Use during pregnancy or breastfeeding:
Carbidopa and levodopa should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether carbidopa and levodopa are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from carbidopa and levodopa, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.