Tofranil-PM
23 June, 2023
Tolterodine
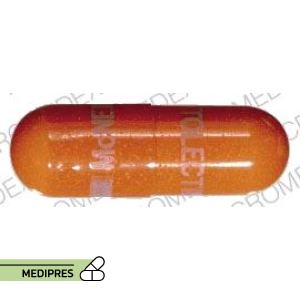
23 June, 2023Tolectin DS
Generic name: Tolmetin sodium
Drug class: Nonsteroidal anti-inflammatory drug (NSAID)
Dosage form: Oral capsule
Route of administration: Oral
Dose: Adults: 400 mg three to four times daily, not to exceed 2,000 mg daily. Pediatric patients (2 years and older): 20 mg/kg/day in divided doses (three or four times daily); usual dose ranges from 15 to 30 mg/kg/day. Doses higher than 30 mg/kg/day have not been studied and are not recommended.
Mechanism of action: Tolmetin inhibits prostaglandin synthetase, leading to reduced prostaglandin E levels, thereby decreasing inflammation, pain, and fever.
Drug usage cases:
- Relief of signs and symptoms of rheumatoid arthritis
- Management of osteoarthritis
- Treatment of acute flares and long-term management of chronic diseases
- Management of juvenile rheumatoid arthritis
Drug contraindications:
- Hypersensitivity to tolmetin or other NSAIDs
- Active gastrointestinal bleeding or ulceration
- Severe renal impairment
- Severe hepatic impairment
- History of asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs
Side effects:
- Gastrointestinal: Nausea, vomiting, diarrhea, abdominal pain, dyspepsia, peptic ulcer, gastrointestinal bleeding, and perforation
- Cardiovascular: Hypertension, palpitations, peripheral edema, and tachycardia
- Central nervous system: Dizziness, drowsiness, fatigue, and headache
- Renal: Azotemia, dysuria, hematuria, nephrotoxicity, oliguria, and pseudoproteinuria
- Hematologic: Blood dyscrasias
- Dermatologic: Pruritus, purpura, rash, and sweating
Warnings:
- Increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke
- Increased risk of serious gastrointestinal adverse events, including bleeding, ulceration, and perforation
- Risk of renal toxicity, especially in patients with pre-existing renal impairment
- Potential for severe skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis
- Use with caution in patients with a history of gastrointestinal disorders, cardiovascular disease, or hypertension
- Monitor blood pressure regularly during treatment
- Discontinue therapy if signs of serious adverse reactions occur
Use during pregnancy or breastfeeding:
Pregnancy Category C: Risk cannot be ruled out. Use during pregnancy should be considered only if the potential benefit justifies the potential risk to the fetus. Avoid use during the third trimester due to potential adverse effects on the fetus. Small amounts of tolmetin are excreted in breast milk; exercise caution when administering to nursing mothers.