Triazolam
23 June, 2023
Triloan II SUIK
23 June, 2023Triglide
Category: T
Description
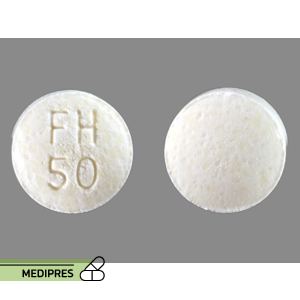
Generic name:
fenofibrate
Drug class:
Fibric acid derivative (fibrate)
Dosage form:
- Oral tablets (micronized)
- Oral capsules (micronized)
Root of administration:
Oral
Dose:
- Typical adult dose: 145 mg once daily
- Dose range: 48–200 mg once daily (formulation-dependent)
- Adjust dosage for renal impairment
Mechanism of action:
Fenofibrate activates peroxisome proliferator-activated receptor alpha (PPAR-α), leading to increased lipoprotein lipase activity, enhanced triglyceride clearance, decreased VLDL production, and modest increases in HDL cholesterol.
Drug usage cases:
- Hypertriglyceridemia
- Mixed dyslipidemia
- Primary hypercholesterolemia
- Hyperlipidemia with risk of pancreatitis
- Off-label: metabolic syndrome-associated dyslipidemia
Drug contra indications:
- Severe renal impairment (e.g., CrCl <30 mL/min)
- Active liver disease or unexplained persistent hepatic dysfunction
- Gallbladder disease (e.g., cholelithiasis)
- Hypersensitivity to fenofibrate or any component of the formulation
- Primary biliary cirrhosis
Side effects:
- Gastrointestinal: nausea, abdominal pain, dyspepsia, constipation, diarrhea
- Hepatic dysfunction: elevated liver enzymes
- Myopathy and rhabdomyolysis (especially with statins)
- Gallstones/cholelithiasis
- Headache, dizziness
- Skin rash, pruritus
- Pancreatitis (rare)
- Increased creatinine levels
Warnings:
- Risk of myopathy/rhabdomyolysis, especially when coadministered with statins
- Monitor liver function tests prior to and during therapy
- Use with caution in patients with renal impairment; dose adjust or avoid if severe
- Caution in patients with pre-existing gallbladder disease
- May increase serum creatinine; monitor renal function
- Potential for cholelithiasis; watch for signs of gallbladder disease
- Not for use in pediatric populations due to safety and efficacy not established
Use during pregnancy or breastfeeding:
Pregnancy: Animal studies have shown adverse fetal effects; safety in human pregnancy not established. Use only if the potential benefit justifies the potential risk to the fetus.
Breastfeeding: Fenofibrate and its metabolites are excreted in breast milk; potential for serious adverse reactions in nursing infants. Discontinue nursing or discontinue drug.