Viracept
23 June, 2023
Virt-PN DHA Softgels
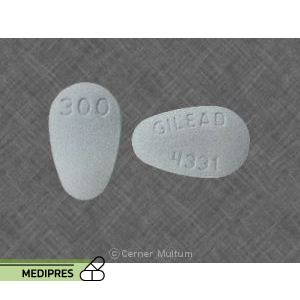
23 June, 2023Viread
Generic name: Tenofovir disoproxil fumarate
Drug class: Nucleotide reverse transcriptase inhibitor (NRTI)
Dosage form: Tablets (150 mg, 200 mg, 250 mg, 300 mg); Granules
Root of administration: Oral
Dose: 300 mg once daily with food; dose adjustments may be necessary in patients with renal impairment
Mechanism of action: Tenofovir disoproxil fumarate is converted to tenofovir in the body, which inhibits reverse transcriptase, an enzyme essential for HIV replication, and DNA polymerase, an enzyme essential for HBV replication, thereby reducing viral load in HIV-1 and HBV infections.
Drug usage cases:
- Treatment of HIV-1 infection in adults and children aged 2 years and older, in combination with other antiretroviral agents
- Treatment of chronic hepatitis B virus (HBV) infection in adults and children aged 2 years and older with compensated liver disease
- Treatment of chronic HBV infection in adults with decompensated liver disease or those who have not responded to lamivudine therapy
Drug contraindications:
- Hypersensitivity to tenofovir disoproxil fumarate or any component of the formulation
- Co-administration with other tenofovir-containing products, including Truvada
Side effects:
- Gastrointestinal disturbances: nausea, diarrhea, vomiting, flatulence
- Headache
- Fatigue
- Abdominal pain
- Renal impairment, including acute renal failure and Fanconi syndrome
- Bone mineral density loss, potentially leading to fractures
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases
Warnings:
- Monitor renal function prior to and during therapy; dose adjustments may be required in patients with renal impairment
- Assess bone mineral density in patients with risk factors for bone loss
- Monitor for signs and symptoms of lactic acidosis and hepatotoxicity
- Discontinue therapy if signs of lactic acidosis or severe hepatotoxicity occur
- Use caution in patients with a history of hepatitis B or C infection; discontinuation may lead to acute exacerbation of hepatitis
- Not recommended for use in combination with other tenofovir-containing products
Use during pregnancy or breastfeeding:
Tenofovir disoproxil fumarate is classified as a Category B3 drug for pregnancy, indicating that studies in animals have shown some evidence of fetal harm, but there are no adequate and well-controlled studies in pregnant women. It should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Tenofovir is excreted in human milk; therefore, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Caution is advised when administering to pregnant or breastfeeding women.