Zavzpret
23 June, 2023
Zemplar Injection
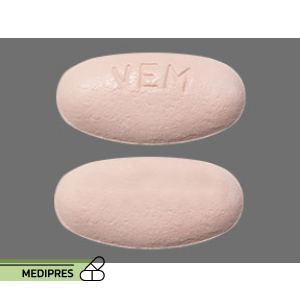
23 June, 2023Zelboraf
Generic name: Vemurafenib
Drug class: Multikinase inhibitors
Dosage form: Tablets (240 mg)
Route of administration: Oral
Dose: 960 mg (four 240 mg tablets) orally every 12 hours with or without food. Treatment continues until disease progression or unacceptable toxicity occurs. A missed dose can be taken up to 4 hours prior to the next dose. Do not take an additional dose if vomiting occurs after administration; continue with the next scheduled dose. Do not crush or chew the tablets.
Mechanism of action: Vemurafenib is a selective inhibitor of the BRAF V600E mutation, a genetic alteration found in certain cancers. By inhibiting this mutated protein, vemurafenib disrupts the MAPK signaling pathway, leading to reduced tumor cell proliferation and survival.
Drug usage cases:
- Unresectable or metastatic melanoma with BRAF V600E mutation
- Erdheim-Chester disease with BRAF V600 mutation
Drug contraindications:
- Hypersensitivity to vemurafenib or any component of the formulation
- Patients with wild-type BRAF melanoma
Side effects:
- Skin reactions: new primary cutaneous malignancies, including cutaneous squamous cell carcinoma and keratoacanthomas
- Arthralgia (joint pain)
- Rash
- Fatigue
- Photosensitivity (increased sensitivity to sunlight)
- Hair loss (alopecia)
- Elevated liver enzymes (transaminases)
- QT interval prolongation
- Hyperkeratosis (thickening of the skin)
- Fever
- Headache
- Diarrhea
- Pruritus (itching)
- Hyperglycemia (elevated blood sugar)
- Hypophosphatemia (low phosphate levels)
- Hypocalcemia (low calcium levels)
- Hypokalemia (low potassium levels)
- Hypomagnesemia (low magnesium levels)
Warnings:
- Monitor for new primary cutaneous malignancies; perform regular skin examinations
- Assess liver function before and during treatment; monitor for signs of hepatotoxicity
- Monitor QT interval; discontinue if QTc > 500 ms or increased by > 60 ms from baseline
- Monitor for signs of hyperkeratosis; consider dose reduction or interruption if severe
- Monitor for signs of ocular toxicity; perform regular eye examinations
- Monitor for signs of hypersensitivity reactions; discontinue if severe reactions occur
- Monitor for signs of cardiotoxicity; assess for arrhythmias and other cardiac events
- Monitor for signs of electrolyte imbalances; correct as necessary
- Monitor for signs of hyperglycemia; manage as appropriate
- Monitor for signs of hypophosphatemia, hypocalcemia, hypokalemia, and hypomagnesemia; correct as necessary
- Monitor for signs of dermatologic toxicities; manage as appropriate
- Monitor for signs of gastrointestinal toxicities; manage as appropriate
- Monitor for signs of hematologic toxicities; manage as appropriate
- Monitor for signs of renal toxicities; manage as appropriate
- Monitor for signs of musculoskeletal toxicities; manage as appropriate
- Monitor for signs of central nervous system toxicities; manage as appropriate
- Monitor for signs of metabolic toxicities; manage as appropriate
- Monitor for signs of endocrine toxicities; manage as appropriate
- Monitor for signs of reproductive toxicities; manage as appropriate
- Monitor for signs of immunologic toxicities; manage as appropriate
- Monitor for signs of hypersensitivity reactions; manage as appropriate
- Monitor for signs of cardiovascular toxicities; manage as appropriate
- Monitor for signs of electrolyte imbalances; manage as appropriate
- Monitor for signs of dermatologic toxicities; manage as appropriate
- Monitor for signs of gastrointestinal toxicities; manage as appropriate
- Monitor for signs of hematologic toxicities; manage as appropriate
- Monitor for signs of renal toxicities; manage as appropriate
- Monitor for signs of musculoskeletal toxicities; manage as appropriate
- Monitor for signs of central nervous system toxicities; manage as appropriate
- Monitor for signs of metabolic toxicities; manage as appropriate
- Monitor for signs of endocrine toxicities; manage as appropriate
- Monitor for signs of reproductive toxicities; manage as appropriate
- Monitor for signs of immunologic toxicities; manage as appropriate
Use during pregnancy or breastfeeding: Vemurafenib may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment and for at least 2 weeks after the last dose. It is not known whether vemurafenib is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants, advise women to discontinue breastfeeding during treatment and for at least 2 weeks after the last dose.